41 fda approved health claims on food labels
Questions and Answers on Dietary Supplements | FDA May 06, 2022 · Among the claims that can be used on dietary supplement labels are three categories of claims that are defined by the FD&C Act and FDA regulations: health claims (claims about the relationship ... FDA Drug Safety Communication: FDA cautions about using ... [03-03-2015] The U.S. Food and Drug Administration (FDA) cautions that prescription testosterone products are approved only for men who have low testosterone levels caused by certain medical ...
FDA Regulation of Cannabis and Cannabis-Derived Products ... In addition, under 21 CFR 530.20, extralabel use of an approved human drug in a food-producing animal is not permitted if an animal drug approved for use in food-producing animals can be used in ...
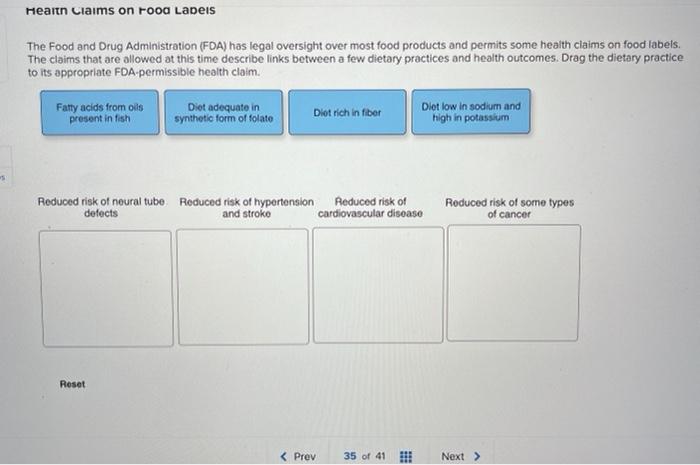
Fda approved health claims on food labels
The FDA Wants to Update the Definition for "Healthy" Claims ... Sep 28, 2022 · A new Food and Drug Administration proposed rule, "Food Labeling: Nutrient Content Claims; Definition of Term Healthy," released on September 28, would offer new guidance to brands who label their products as "healthy" options. The FDA is looking to regulate the use of the word because of the rise of diet-related illnesses in the U.S. Pet Food Labels - General | FDA The term "natural" is often used on pet food labels. AAFCO has developed a feed term definition for what types of ingredients can be considered “natural” and “Guidelines for Natural Claims ... Is It Really 'FDA Approved'? - Food and Drug Administration May 10, 2022 · The FDA is responsible for protecting public health by regulating human drugs and biological products, animal drugs, medical devices, tobacco products, food (including animal food), cosmetics, and ...
Fda approved health claims on food labels. Changes to the Nutrition Facts Label | FDA - U.S. Food and ... Mar 07, 2022 · Manufacturers with $10 million or more in annual sales were required to update their labels by January 1, 2020; manufacturers with less than $10 million in annual food sales were required to ... Pet Food | FDA - Food and Drug Administration For more information about labeling requirements, see Pet Food Labels - General. FDA also reviews specific claims on pet food, such as “maintains urinary tract health,” “low magnesium ... Is It Really 'FDA Approved'? - Food and Drug Administration May 10, 2022 · The FDA is responsible for protecting public health by regulating human drugs and biological products, animal drugs, medical devices, tobacco products, food (including animal food), cosmetics, and ... Pet Food Labels - General | FDA The term "natural" is often used on pet food labels. AAFCO has developed a feed term definition for what types of ingredients can be considered “natural” and “Guidelines for Natural Claims ...
The FDA Wants to Update the Definition for "Healthy" Claims ... Sep 28, 2022 · A new Food and Drug Administration proposed rule, "Food Labeling: Nutrient Content Claims; Definition of Term Healthy," released on September 28, would offer new guidance to brands who label their products as "healthy" options. The FDA is looking to regulate the use of the word because of the rise of diet-related illnesses in the U.S.
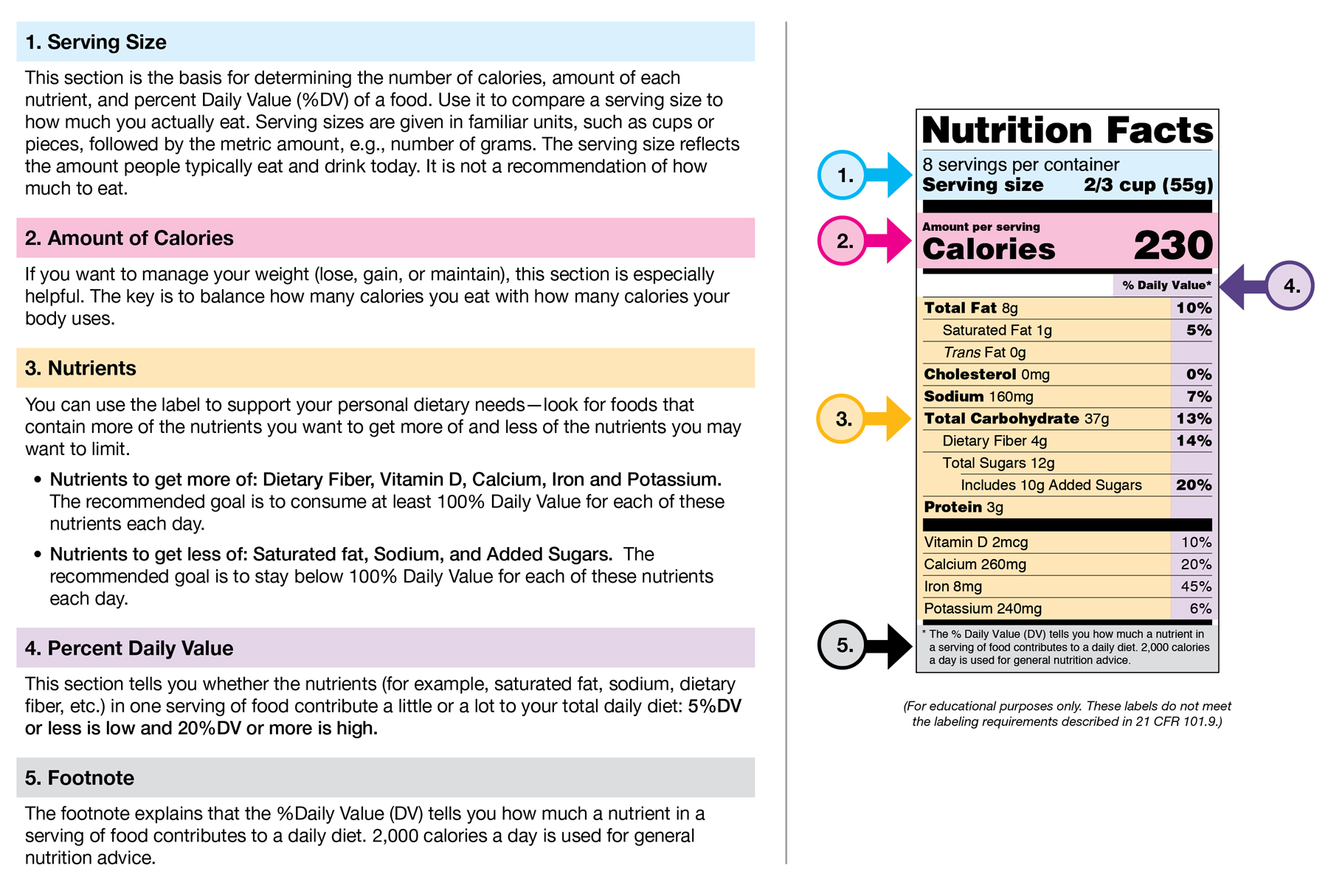
:no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/3652082/healthy-choice.0.png)












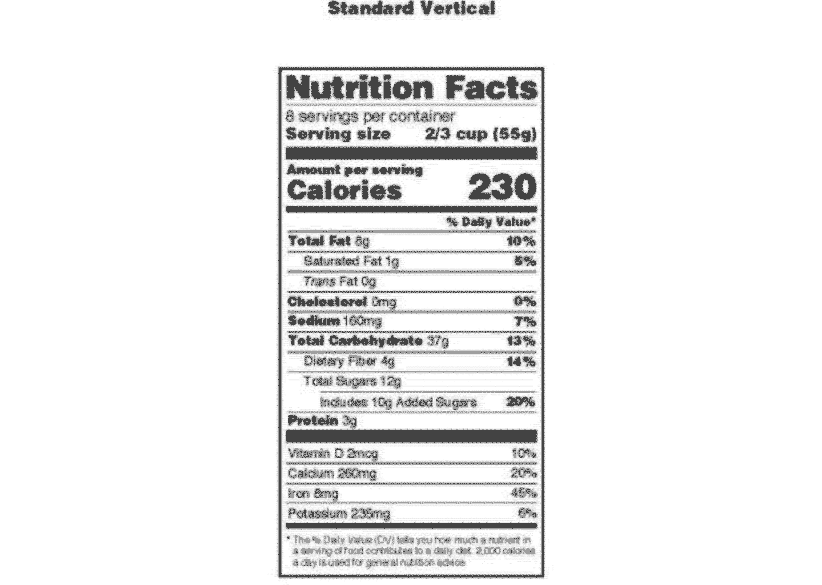



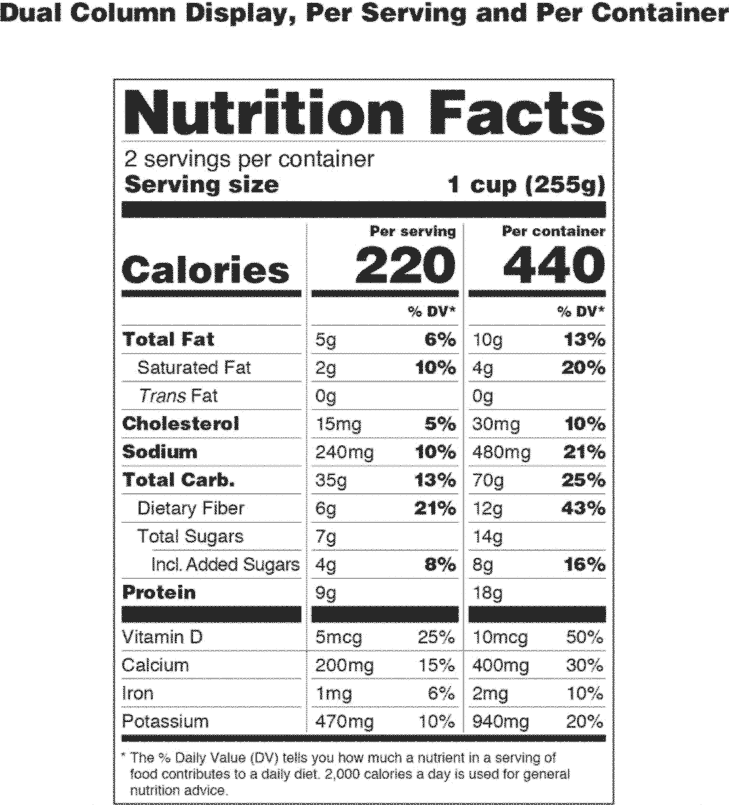













:max_bytes(150000):strip_icc()/GettyImages-1350430166-ffcfb825372249f490fd19ea9cab075e.jpg)
Post a Comment for "41 fda approved health claims on food labels"